Composition
Once upon a time, billions of years ago, there was a whole bunch of atoms floating around the universe. Slowly those atoms and molecules came together and formed what we call the Earth. Now the Earth is a big ball of matter that circles the Sun once a year. What's inside the planet? Most of the Earth is composed of the mantle and the core.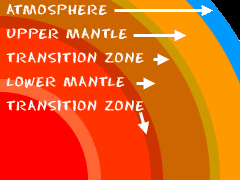 The rules of density were in action when the Earth and its matter came together. Those rules explain how the heavier substances moved towards the middle and the lighter substances wound up on top. It's just like sand sinking to the bottom of a water glass.
The rules of density were in action when the Earth and its matter came together. Those rules explain how the heavier substances moved towards the middle and the lighter substances wound up on top. It's just like sand sinking to the bottom of a water glass.
The Mantles
Scientists break the mantle into two pieces. They have the upper mantle and the lower mantle. There are very small differences between the two layers. The upper mantle has Olivine (a very special rock), compounds with silicon dioxide, and a substance called Peridotite.The lower mantle is more solid than the upper mantle. It has a lot of that Olivine rock, iron, magnesium, and many silicate compounds (those are ones with SiO2).
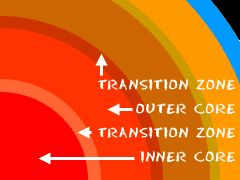
The Cores
Don't get confused with the next part. There aren't two cores. Like the mantle, scientists break the core into two layers. First is the outer core. Imagine this huge sphere of molten iron (Fe), floating and flowing around the inner core. It's really a liquid. It's extremely hot and under a lot of pressure. Especially important is the idea that the outer core creates the Earth's magnetic field. It's a huge magnet in the center of the planet.The inner core is under even more pressure. Even though it's just as hot as the outer core, there is such high pressure that it stays solid. Remember those rules about density? Well the most dense, most compact, heaviest matter is found in the inner core. It's not pure iron like the outer core. It has compounds that use the element iron.
Or search the sites for a specific topic.
- Overview
- Composition
- Magnetic Field
- Structure
- Rock Types
- Tectonics
- Faulting
- Earthquakes
- Volcanoes
- More Topics

How to Build a Planet (NASA/Spitzer Video)

Useful Reference Materials
Encyclopedia.com (Planet Earth):http://www.encyclopedia.com/topic/planet_Earth.aspx
Wikipedia (Structure of the Earth):
http://en.wikipedia.org/wiki/Structure_of_the_Earth
Encyclopædia Britannica (Interior of the Earth):
http://www.britannica.com/EBchecked/topic/175962/Earth/54199/The-interior?anchor=ref514518





